PPM Calculator
Calculate PPM
How do I calculate my ratio setting and ppm?
We have dedicated this portion of our website to provide our distributor network with additional product support.
When using a water-driven injector to dispense the chemical, it is imperative that the chemical be water-soluble.
These guidelines only apply to water-soluble chemicals. Do not use if mixing liquid chemicals.
If you have determined that the product is water soluble, the next step is to identify the chemicals solubility limit. If product is added beyond the solubility limit, it will not dissolve, but will settle out in the solution container. This causes clogging in the injector, and the proper dosage of solution will not be dispensed. Premature failure of the seals, o-rings, dosage piston, inner cylinder and check poppet can also occur.
The next step is to determine the amount of chemical required for the application. The user must decide what parts per million (ppm) of chemical is right for the particular application. Neither the chemical manufacturer nor the injector manufacturer is responsible for specifying a ppm for the user’s application. The best way to determine fertilizer needs is to have the soil or foliage tested. Contact your local agricultural extension agency or other expert for assistance.
For other non-horticultural applications, contact an authority for the application.
Once you know the desired ppm, you can usually find a pre-calculated chart on the back of the chemical bag or container listing the equivalent ratio, percentage or ounces per gallon. If you don’t have a chart, you can still determine how to correctly mix the chemical to the amount of water in your container. The following outlines ways to figure the amount of chemical injected per gallon of water.
Method A gives a formula for finding the pounds of chemical or fertilizer per one gallon of water delivered to the source, if you know the ppm required.
Method B outlines how to use the formula from Method A to calculate changing injection ratios once your solution is mixed. For both methods four factors are critical: 1) the desired ppm or the weight of the chemical added; 2) the % of active ingredient; 3) the injection ratio and 4) the tank size in gallons.
Method A:
To determine the pounds (or ounces) required if you know the ppm of chemical per gallon of water needed follow the steps below.
The following example is a fertilizer application, but the principle applies to any chemical solution.
Example: You want to fertigate at 150 ppm of nitrogen using a 20-10-20 soluble fertilizer. You have your injector set at a 1:100 ratio. How much fertilizer should be put in the solution tank?
Ounces of fertilizer = 150 ppm x 100/75 x 20 Answer = 10 ounces of fertilizer per gallon of water.
The 150 = the recommeded ppm for the crop; the 100 = the injection ratio; the 20 = the % of active ingredient; the 75 is a constant conversion factor used for finding ppm (1 ounce of any 100 percent soluble fertilizer or chemical in 100 gallons of water always equals 75 ppm). The tank size or gallons is one gallon here. If you have a tank size of more than one gallon, multiply your answer by the number of gallons.
Method B:
To calculate what injection ratios would provide other ppms of the same chemical mixture, follow the steps below.
If mixing the fertilizer for various ppms, you will need to choose one ppm as your base for a 1:100 injection ratio and then calculate the other ratio settings for the other ppms.
Example: If you have various fertilizers such as 20-20-20, 10-52-17, etc., choose nitrogen (the second number) as the chemical to determine your ppm.
Formula: Recommended ppm x injection ratio/75 (constant) x % fertilizer. Choose 20-20-20 and solve for 50 ppm, 100 ppm and 200 ppm. Select 100 ppm as your base. You could choose 50 or 200, but choosing 100 simplifies calculations. Also, choose 1:100 as your injection ratio. You could choose any ratio setting to find the ounces per gallon needed, but choosing 1:100 makes it much easier to calculate your settings.
100 ppm x 100/75 x 20 = 6.67 oz per gallon. Answer = 6.67 oz per gallon to obtain 100 ppm at a 1:100 ratio setting.
If you are using an 8 gallon container, multiple 6.67 oz. by 8 = 53.3 oz or 3.33 lbs. per 8 gallons, and so on for other gallon containers.
Solve for Ratio at 50 ppm. Since you now know that you mixed 6.67 oz. per gallon of water, the missing variable is the injection ratio or setting that you need to mix the solution at 50 ppm, so R = injection ratio.:
Step 1 50 ppm x R/75 x 20 = 6.67 oz.
Step 2 50R/1500 = 6.67
Step 3 50R = 6.67 x 1500
Step 4 50R = 10,005
Step 5 R = 10,005 50
Step 6 R = 200
You now know that you need to adjust your ratio from 1:100 to 1:200.
Solve for Ratio at 200 ppm. Follow the exact steps above, but substitute “200” ppm for “50” ppm.
You can increase or decrease your ppm by adjusting the ratio of the injector while it is in operation. The Dosmatic® injector allows you to simply leave the solution container as it is and adjust the injector ratio instead.
Below is an example of how to adjust your ratio to increase your ppm. Although the example is for horticulture, the method holds true for animal health and industrial applications.
Example:
You have added 13.4 ozs. of fertilizer (20-20-20) to one gallon of water using a 1:200 injector ratio to achieve 100 ppm of Nitrogen. You have a 50 gallon tank so you multiply 13.4 ozs. x 50 = 670 ozs. or 41.875 lbs. and add this to 50 gallons of water in your solution tank. Now your plants are mature, and you want to fertigate at a ppm of 200. You simply adjust the injector ratio from 1:200 (or 0.5%) to 1:100 (or 1%) and your ppm is now 200! (Remember, the higher the ppm or % required, the lower the ratio number).
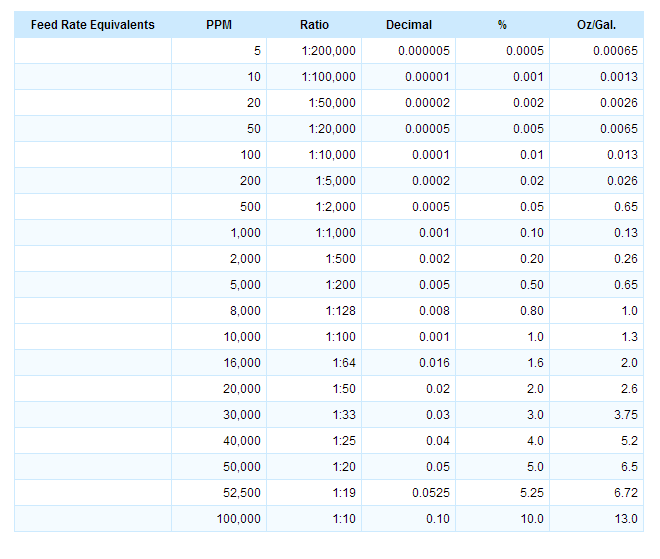
Calculations for Feed Rate Equivalent